[ROWS] 932
[FIELDS] 17
33 Drug Rebate Program
Product Data for Newly Reported Drugs in the Medicaid Drug Rebate Program
The Table below, updated weekly, contains newly reported, active covered outpatient drugs which were reported by participating drug manufacturers since the last quarterly update of the Drug Products in the Medicaid Drug Rebate Program (MDRP) database. Each file on this table represents a snapshot of data in the system and is not updated by subsequent changes. Once the covered outpatient drugs in each of these files appear in the quarterly MDRP database, the file will be removed from this table. States can utilize these files to identify newly reported covered outpatient drugs.
Metadata
Dictionary
1 ndc1: Labeler Code - First segment of National Drug Code (NDC1) that identifies the manufacturer, labeler, relabeler, packager, repackager or distributor of the drug.
2 ndc2: Product Code - Second segment of National Drug Code (NDC2)
3 ndc3: Package Size - Third segment of National Drug Code (NDC3)
4 labeler_name: Labeler Name: Name of labeler as it appears on the signed rebate agreement.
5 labeler_status: Labeler Status: Active or Terminated
6 fda_name: The FDA Name should reflect the drug's name as reported to FDA; therefore, the FDA Name reported to CMS should correspond to the drug name on file with the FDA.
7 cod_status: Covered Outpatient Drug (COD) Status: A category that identifies whether or not a product meets the statutory definition of a covered outpatient drug in accordance with sections 1927(k)(2) to 1927(k)(4) of the Social Security Act. Numeric values; 8-digit field; format: MMDDYYYY. Valid Values: 01 = Abbreviated New Drug Application (ANDA) 02 = Biological License Application (BLA) 03 = New Drug Application (NDA) 04 = NDA Authorized Generic 05 = DESI 5* - LTE/IRS drug for all indications 06 = DESI 6* - LTE/IRS drug withdrawn from market 07 = Prescription Pre-Natal Vitamin or Fluoride 08 = Prescription Dietary Supplement/Vitamin/Mineral (Other than Prescription Pre-Natal Vitamin or Fluoride) 09 = OTC Monograph Tentative 10 = OTC Monograph Final 11 = Unapproved Drug - Drug Shortage 12 = Unapproved Drug - Per 1927(k)(2)(A)(ii) 13 = Unapproved Drug - Per 1927(k)(2)(A)(iii) Spaces = The Labeler has not reported this field to the Medicaid Drug Rebate Program. * NDCs with a COD Status of DESI 5/6 are not eligible for coverage or rebates under the Medicaid Drug Rebate Program.
8 fda_application_number: FDA Application Number/OTC Monograph Number: For drugs with a COD status of ANDA, BLA, NDA, or NDA Authorized Generic, this is the application number (assigned by the FDA for approval to market a drug or biological in the United States) under which the NDC is currently marketed. Numeric values; 7-digit field; padded with leading zeros as needed. For drugs with a COD status of OTC Monograph Tentative or Final, this is the FDA's regulatory citation for the OTC. Alpha-numeric values; 7-digit field. For drugs with a COD Status of OTC Monograph Final, the first four characters are a constant of PART; the last three characters are the numeric values for the appropriate regulatory citation for the product (e.g., 225). For drugs with a COD Status of OTC Monograph Tentative, the first four characters are a constant of PART; the last three characters are the numeric values for the appropriate regulatory citation for the product, or three zeros if a Monograph Number is not available. For drugs with a COD Status other than ANDA, BLA, NDA, NDA Authorized Generic, OTC Monograph Final, or OTC Monograph Tentative, the FDA Application Number/OTC Monograph Number field should be zero-filled.
9 drug_category: Drug Category: This field indicates whether the drug is single source (S), innovator multiple source (I), or non-innovator multiple source (N). 1-character field. Valid Values: N = Non-innovator multiple source S = Single source I = Innovator multiple source
10 drug_type: Drug Type: Identifies a drug as prescription (Rx) or Over-the-Counter (OTC). Numeric values; 1-digit field. Valid Values: 1 = Rx 2 = OTC
11 line_extension: Line Extension Drug Indicator: Identifies whether a product is a line extension drug as defined in Section 1927(c)(2)(C) of the Social Security Act, including whether the drug is excluded from the statutory definition of a line extension on the basis of being an abuse-deterrent formulation (ADF). If a labeler is seeking an ADF exclusion, a value of R will appear in this field. Note: If a Line Extension Drug Indicator has not been reported for an NDC, this field will be blank. Valid Values: Y = Yes N = No (i.e., neither LE nor ADF) R = Request for ADF Exclusion E = Excluded (Due to ADF) Note: This value may only be assigned by CMS
12 fda_approval_date: FDA Approval Date: NDA (including Authorized Generic), ANDA or BLA approval date. For covered outpatient drugs for which the FDA does not require approval, the FDA Approval Date will be 09/30/1990, or, if the drug was first marketed after 09/30/1990, the actual date the drug was first marketed.
13 market_date: Market Date: For S, I, and N drugs marketed under an FDA-approved application (e.g. BLA, NDA, ANDA), the earliest date the drug was first marketed under the application number by any labeler. For drugs marketed without an FDA-approved application (e.g. OTC monograph, unapproved drug), the earliest date the drug was first marketed by any labeler. For all drugs (i.e., those marketed with or without an FDA-approved application), if a drug was purchased or otherwise acquired from another labeler, the Market Date should be equal to the Market Date of the original product. If a Market Date falls on a date that is earlier than 9/30/1990, CMS will change it to 9/30/1990 in the Medicaid Drug Programs (MDP) system since dates earlier than the start of the Drug Rebate Program have no bearing on the program.
14 unit_type: Unit Type: One of the 10 unit types by which a drug may be dispensed. 3-character field; left-justified; blank-filled for Unit Type values with fewer than 3 characters. Valid Values: AHF = Injectable Anti-Hemophilic Factor CAP = Capsule EA = Each GM = Gram MCI = Millicurie ML = Milliliter UCI = Microcurie SUP = Suppository TAB = Tablet TDP = Transdermal patch
15 unit_per_package_size: Units Per Package Size: The total number of units in the smallest dispensable amount for the 11-digit NDC. Numeric values; 11-digit field: 7 whole numbers, the decimal point (.') and 3 decimal places; right-justified; zero-padded for UPPS values with fewer than 11 digits.
16 therapeutic_equivalent_code: Therapeutic Equivalence Code (TEC): FDA-assigned Therapeutic Equivalence Codes as found in the FDA's Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Alpha-numeric values; 2-digit field. Valid Values: AA = Products in Conventional Dosage Forms Not Presenting Bioequivalence Problems AB = Products Meeting Necessary Bioequivalence Requirements assigned an FDA TEC of AB, or AB1 through AB9 AN = Solutions and Powders for Aerosolization AO = Injectable Oil Solutions AP = Injectable Aqueous Solutions and, in Certain Instances, Intravenous Non-Aqueous AT = Topical Products BC = Extended-Release Dosage Forms (Capsules, Injectables, and Tablets) BD = Active Ingredients and Dosage Form With Documented Bioequivalence Problems BE = Delayed-Release Oral Dosage Forms BN = Products in Aerosol-Nebulizer Drug Delivery Systems BP = Active Ingredients and Dosage Forms with Potential Bioequivalence Problems BR = Suppositories or Enemas That Deliver Drugs for Systemic Absorption BS = Products Having Drug Standard Deficiencies BT = Topical Products with Bioequivalence Issues BX = Drug Products for Which the Data Are Insufficient To Determine Therapeutic Equivalence NR = Not Rated
17 5i_indicator: The 5i Drug Indicator specifies whether or not the product is a 5i drug (i.e., inhaled, instilled, implanted, injected, or infused). (Y or N)
18 purchased_product_date: Purchased Product Date (PPD): The date the company currently holding legal title to the NDC first markets the drug under this NDC (this date can result, for example, from the purchase of an NDC from one company by another company, the re-designation of an NDC from one of a company's labeler codes to another of that same company's labeler codes, cross-licensing arrangements, etc.). Zero or blank-filled for drugs without Purchased Product Dates.
19 coverage_effective_date: The Coverage Effective Date field reflects the date on which a product is first eligible for coverage under the MDRP.
20 drug_termination_date: Termination Date: The date on which the drug was withdrawn from market or the drug's last lot expiration date.
21 drug_reactivation_date: Reactivation Date: The date on which a terminated product is re-introduced to the market.
22 date_reported_to_cms: Date Reported to CMS: The date on which the product was initially reported to CMS via MDP
1 year: NA
2 quarter: NA
3 labeler_name: The corporate name of the entity identified by the labeler code.
4 ndc: The National Drug Code (NDC) is a numerical code maintained by the FDA that includes the labeler code, product code, and package code. The NDC is an 11-digit code.
5 labeler_code: The first segment of the National Drug Code that identifies the labeler.
6 product_code: The second segment of the National Drug Code that identifies the product.
7 package_size_code: The third segment of the National Drug Code that identifies the package size.
8 drug_category: This field indicates whether the drug is single source (S), innovator multiple source(I), or non- innovator multiple source (N). Valid Values: N = Non-innovator multiple source S = Single source I = Innovator multiple source
9 drug_type_indicator: This field identifies a drug as prescription (Rx) or Over-the-Counter (OTC). Valid values: 1 = Rx 2 = OTC
10 termination_date: The date on which the drug was withdrawn from market or the drug's last lot expiration date.
11 unit_type: One of the 8 unit types by which a drug can be dispensed. Valid Values: AHF = Injectable Anti-Hemophilic Factor CAP = Capsule SUP = Suppository GM = Gram ML = Milliliter TAB = Tablet TDP = Transdermal
12 units_per_pkg_size: The total number of units in the smallest dispensable amount for the 11-digit NDC.
13 fda_approval_date: The NDC or monograph approval date.
14 market_date: For S, I, and N drugs marketed under an FDA-approved application (e.g. BLA, NDA, ANDA), the earliest date the drug was first marketed under the application number by any labeler. For drugs marketed without an FDA-approved application (e.g. OTC monograph, unapproved drug), the earliest date the drug was first marketed by any labeler. For all drugs (i.e., those marketed with or without an FDA-approved application), if a drug was purchased or otherwise acquired from another labeler, the Market Date should be equal to the Market Date of the original product. Thus, the Market Date of a drug is frequently not the date on which a labeler began marketing the drug, but may be a much earlier date. If a Market Date falls on a date that is earlier than 9/30/1990, CMS will change it to 9/30/1990 in both the Medicaid Drug Rebate (MDR) system and the Drug Data Reporting for Medicaid (DDR) system since dates earlier than the start of the Drug Rebate Program have no bearing on the program. Numeric values, 8-digit field, format: MM/DD/YYYY.
15 fda_therapeutic_equivalence_code: The Therapeutic Equivalence Code (TEC) reported to CMS should correspond to the FDA Therapeutic Equivalence (TE) Code assigned to a product by the FDA. More information regarding FDA TE Codes can be found at the Orange Book at: http://www.accessdata.fda.gov/scripts/cder/ob/default.cfm or Drugs@FDA at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Addlsearch_drug name. It is important to note that while the FDA generally assigns a two letter TE Code value (e.g., AB) to products, in some cases the value is two letters plus a number (e.g., AB1). Since DDR restricts values reported in the TEC field to two characters, some FDA TE Codes need to be converted to a two-character format. Specifically, if the FDA assigned TE Code is AB1, AB2, etc., the corresponding TEC value should be reported in DDR as A1, A2, etc. Alpha-numeric values, 2 character field. Valid Values: A1 A6 AB BC BR A2 A7 AN BD BS A3 A8 AO BE BT A4 A9 AP BN BX A5 AA AT BP NR (Not Rated)
16 fda_product_name: The drug's name as approved by the FDA.
17 clotting_factor_indicator: In accordance with section 1927(c)(1)(B)(iii) of the Social Security Act, an indicator which identifies a Single Source or Innovator Multiple Source drug as a clotting factor for which a separate furnishing payment is made under section 1842(o)(5) of the Act. Valid values: Y = Yes N = No
18 pediatric_indicator: In accordance with section 1927(c)(1)(B)(iii) of the Social Security Act, an indicator which identifies a Single Source or Innovator Multiple Source drug approved by the FDA exclusively for pediatric indications for patients in the FDA-defined pediatric age group (i.e., birth to 16 years). Valid values: Y = Yes N = No
19 package_size_intro_date: The date the package size is first available on the market. If the product was purchased from another company, the Package Size Introduction Date should equal the date the package size is first available on the market under the labeler code of the company currently holding legal title to the NDC.
20 purchased_product_date: The date on which the company currently holding legal title to the NDC first markets the drug under this NDC (this date can result, for example, from the purchase of an NDC from one company by another company, the re-designation of an NDC from one of a company's labeler codes to another of that same company's labeler codes, cross-licensing arrangements, etc).
21 cod_status: A category that identifies whether or not a product meets the statutory definition of a covered outpatient drug in accordance with sections 1927(k)(2) to 1927(k)(4) of the Social Security Act. Valid Values: ' ' (Spaces) = The Labeler has not reported this field to the Medicaid Drug Rebate Program. '01' = Abbreviated New Drug Application (ANDA) '02' = Biological License Application (BLA) '03' = New Drug Application (NDA) '04' = NDA Authorized Generic '05' = DESI 5* - LTE/IRS drug for all indications '06' = DESI 6* - LTE/IRS drug withdrawn from market '07' = Prescription Pre-Natal Vitamin or Fluoride '08' = Prescription Dietary Supplement/Vitamin/Mineral (Other than Prescription Pre-Natal Vitamin or Fluoride) '09' = OTC Monograph Tentative '10' = OTC Monograph Final '11' = Unapproved Drug - Drug Shortage '12' = Unapproved Drug - Per 1927(k)(2)(A)(ii) '13' = Unapproved Drug - Per 1927(k)(2)(A)(iii) *NDCs with a COD Status of DESI 5/6 are not eligible for coverage or rebates under the Medicaid Drug Rebate Program.
22 fda_application_number: For drugs with a COD status of ANDA, BLA, NDA, or NDA Authorized Generic, this is the seven-digit application number that is assigned by the FDA for approval to market a generic drug or new drug in the United States For drugs with a COD status of OTC Monograph Tentative or Final, this is the FDA's August 2018 Page 5 of 5 regulatory citation for the OTC For drugs with a COD Status of OTC Monograph Final, the first four characters are a constant of PART; the last three characters are the numeric values for the appropriate regulatory citation for the product (e.g., '225') For drugs with a COD Status of OTC Monograph Tentative, the first four characters area constant of 'PART'; the last three characters are the numeric values for the appropriate regulatory citation for the product, or 3 zeroes if a Monograph Number is not available For drugs with a COD Status other than ANDA, BLA, NDA, NDA Authorized Generic, OTC Monograph Final, or OTC Monograph Tentative, the FDA Application No./OTC Monograph No. field will be zero-filled For drugs where a COD Status has never been reported to CMS, the FDA Application Number/OTC Monograph Number field will be padded with spaces.
23 reactivation_date: The date on which a terminated product is re-introduced to the market.
24 line_extension_drug_indicator: Identifies whether or not a product is a line extension drug as defined inSection 1927(c)(2)(C) of the Social SecurityAct, including whether the drug is excluded from the statutory definition of a line extension on the basis of being an abuse-deterrent formulation (ADF). Valid Values: Y = Yes N = No (i.e., neither LE nor ADF) R = Request for ADF Exclusion E = Excluded (Due to ADF) (NOTE: This value may only be assigned by CMS)
Endpoint
# A tibble: 932 × 17
ndc1 ndc2 ndc3 ndc11 fda_nameproduct_name cod_status
,
# drug_category , drug_type , unit_type , upps ,
# line_extension , tec_code , fda_approval_date ,
# market_date , termination_date , date_transferred
Overview
obs: 932
cols: 17
----- Numeric -----
col n_missng p_complt n_unique mean p0 p25 p50 p75 p100 iqr sd hist
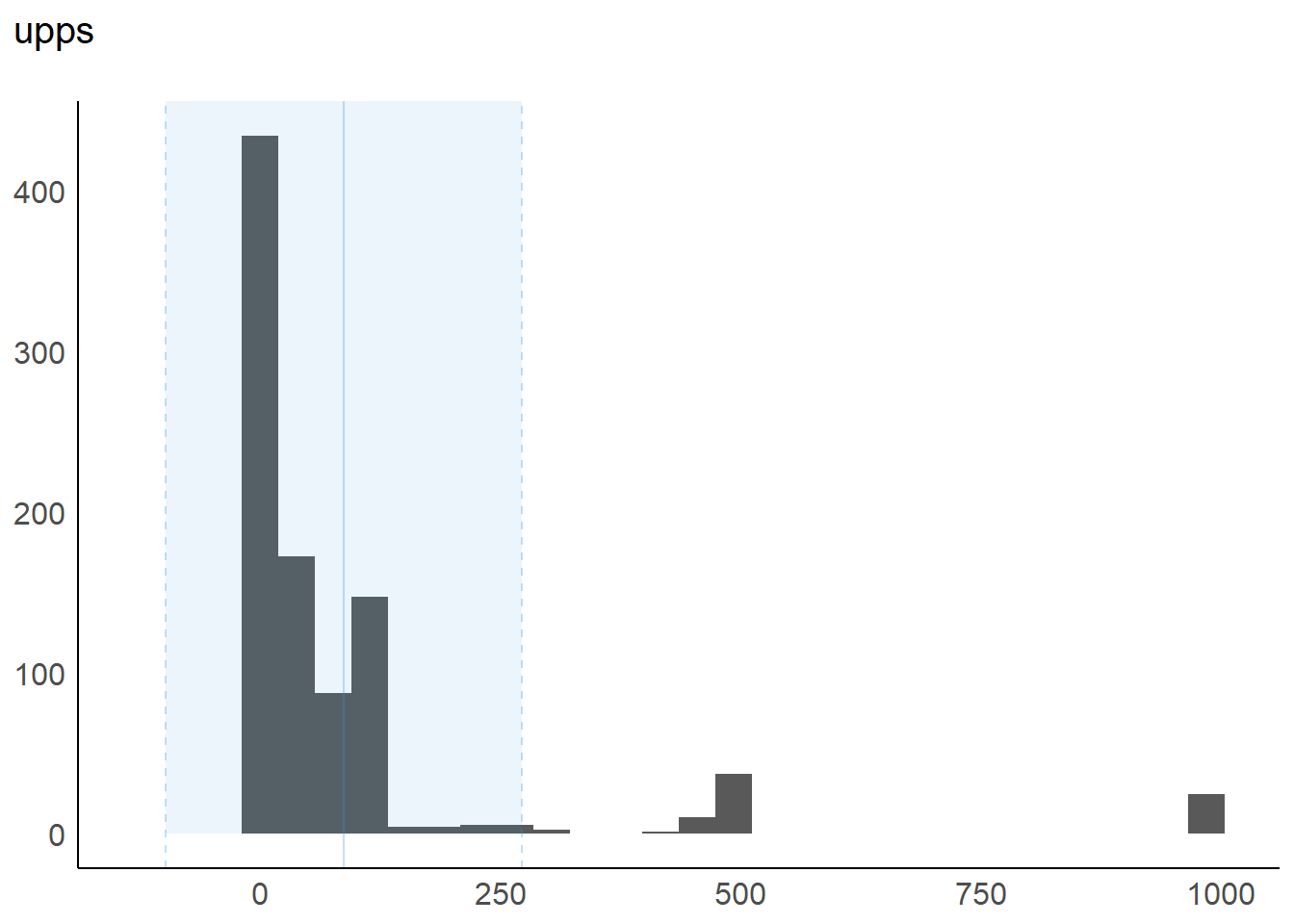
1 upps 0 1 71 86.6 0.14 1 25 100 1000 99 185 ▇▁▁▁▁
----- Dates -----
col n_missng p_complt n_unique min max
1 fda_approvl_dt 0 1 367 1990-09-30 2021-09-15
2 market_date 0 1 367 1990-09-30 2021-09-15
3 termination_dt 932 0 0
4 date_transfrrd 932 0 0
----- Categorical -----
col n_missng p_complt n_unique n_levels
1 ndc1 0 1 170 NA
2 ndc2 0 1 576 NA
3 ndc3 0 1 76 NA
4 ndc11 0 1 932 NA
5 fda_nmprdct_nm 0 1 779 NA
6 cod_status 0 1 9 9
7 fd_pplctn_nm__ 0 1 385 NA
8 drug_category 0 1 3 3
9 drug_type 0 1 2 2
10 unit_type 0 1 8 8
11 line_extension 932 0 0 NA
12 tec_code 0 1 10 10
min
1 00006
2 0001
3 00
4 00006533101
5 2.5% Dextrose and 0.45% Sodium Chl Liquid 25 g 1000mL
6 01
7
8 I
9 1
10 AHF
11
12 A1
max
1 78206
2 9398
3 99
4 78206018201
5 micafungin sodium 20mg/ml 1 5ml vial
6 12
7 PART356
8 S
9 2
10 TDP
11
12 NR
Variable | Mean | SD | IQR | Range | Skewness | Kurtosis | n | n_Missing
-----------------------------------------------------------------------------------------
upps | 86.62 | 185.27 | 99 | [0.14, 1000.00] | 3.60 | 13.55 | 932 | 0