[ROWS] 1965434
[PAGES] 246
[FIELDS] 14
32 ACA Federal Upper Limits
Affordable Care Act Federal Upper Limits (FUL) based on the weighted average of the most recently reported monthly average manufacturer price (AMP) for pharmaceutical and therapeutically equivalent multiple source drug products that are available for purchase by retail community pharmacies on a nationwide basis.
Metadata
Dictionary
1 product_group: The following methodology forms a Product Group: Include all innovator (I) and non-innovator (N) pharmaceutically and therapeutically equivalent (A-rated) multiple source drugs when calculating the weighted average of monthly AMPs in a FUL Product Group; Will not include single source (S) drugs when calculating the weighted average of monthly AMPs in a FUL Product Group; Will not include formulations of the drug that are not rated by the FDA as pharmaceutically and therapeutically equivalent to the reference listed drug, (A-rated) in the calculation of the weighted average of monthly AMPs, or apply the FUL to those formulations that are not A-rated, e.g., B-rated drugs; Will use the monthly AMP and AMP units reported and certified by manufacturers to calculate the weighted average of monthly AMPs in a FUL Product Group; and, Will not include the AMP of a terminated drug in the weighted average of monthly AMPs.
2 ingredient: The order of ingredients presented in the product labeleling.
3 strength: The strengths presented in the product labeleling.
4 dosage: The dosage form of the drug.
5 route: The route of administration of the drug.
6 mdr_unit_type: The unit type reported to the CMS Medicaid Drug Rebate Program.
7 weighted_average_amps: The calculated weighted average of the monthly amps for the group.
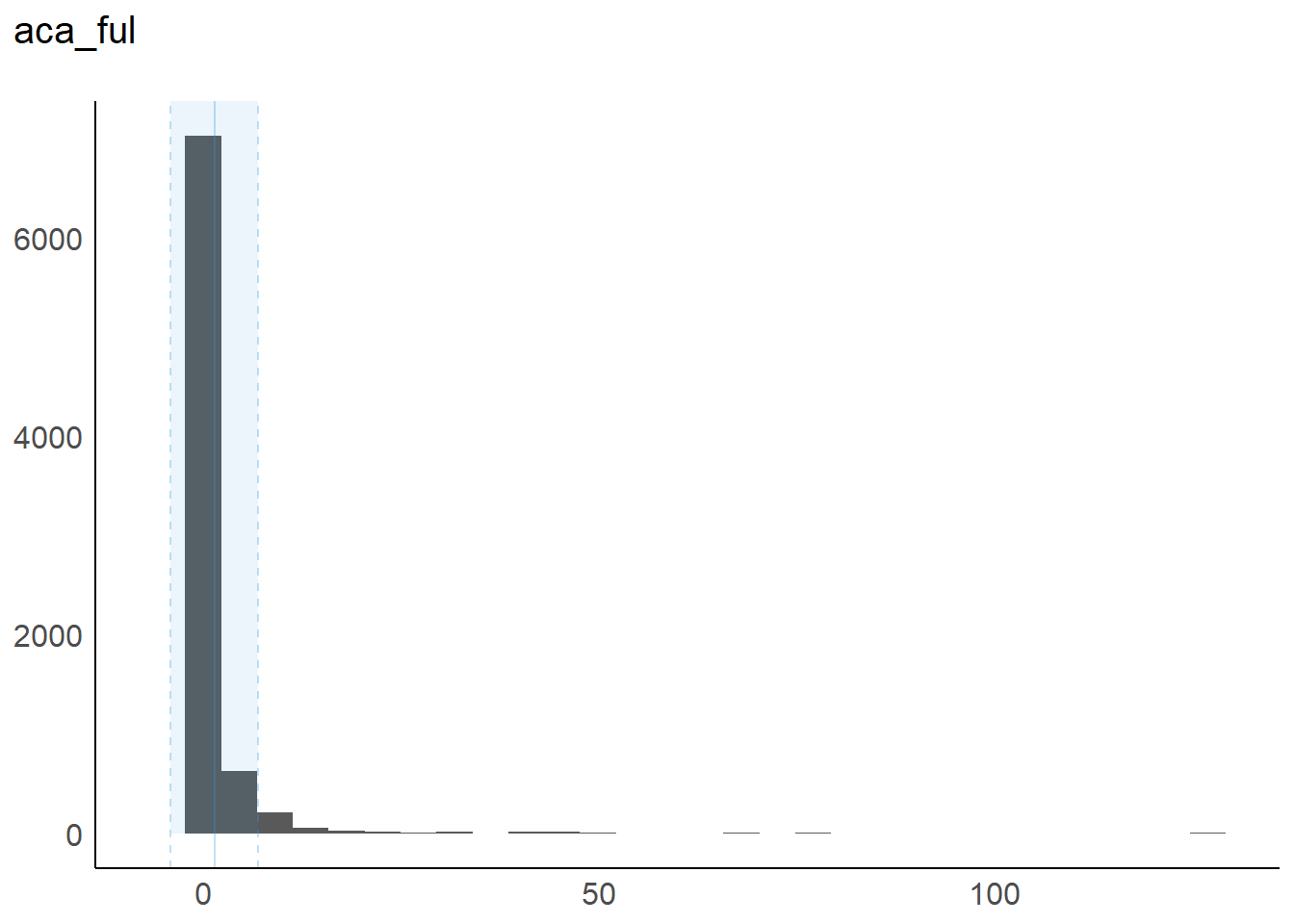
8 aca_ful: The calculated Federal Upper Limit (FUL) for the group.
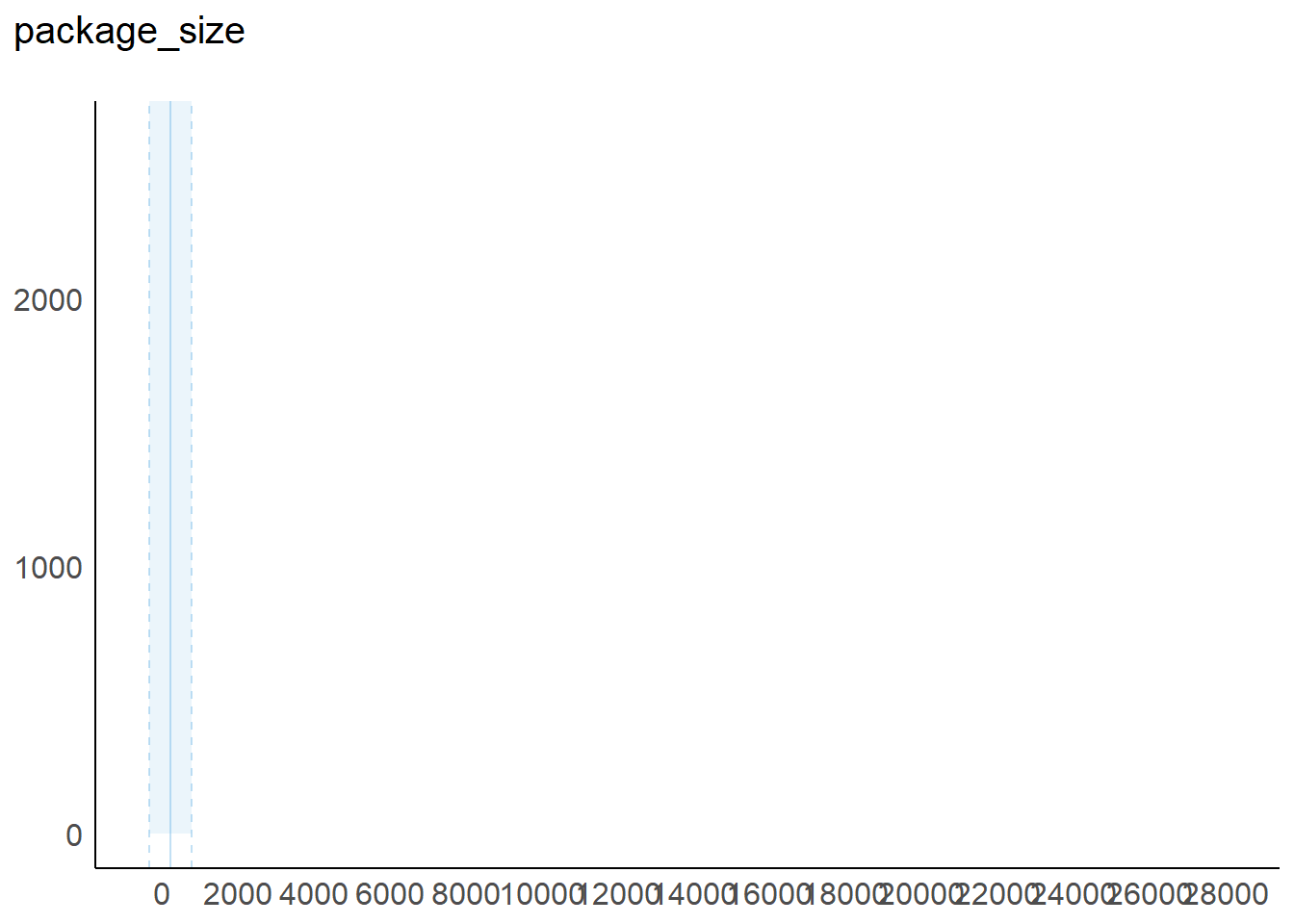
9 package_size: Identifies the number of billing units in the package quantity from which the pharmacist dispenses.
10 ndc: The National Drug Code (NDC) is a numerical code maintained by the FDA that includes the labeler code, product code, and package code.
11 arated: Identifies if the drug is an A-rated drug, (Yes or No).
12 multiplier_greater_than_175_percent_of_weighted_avg_of_amps: ACA FUL equals the most current average retail community pharmacies' acquisition cost (NADAC) = Y ACA FUL equals 175 percent of the weighted average of the most recently reported monthly AMPs = N Note: All NDC-11s for a FUL Product Group are included in this file; however, only the (I) and (N), A-rated, multiple source drugs are used to calculate the weighted average of the most recently reported monthly AMPs in a FUL Product Group.
13 year: The year in which the FUL was calculated.
14 month: The month in which the FUL was calculated.
Data
# A tibble: 8,000 × 14
product_group ingredient strength dosage route mdr_unit_type aca_ful
, ndc , arated , year ,
# month , amp_wt_avg , gt175_amp
obs: 8000
cols: 14
----- Numeric -----
col n_missng p_complt n_unique mean p0 p25 p50
1 aca_ful 0 1 1234 1.43 0.0033 0.099 0.25
2 package_size 0 1 71 227.45 1 30 100
3 arated 0 1 2 0.99 0 1 1
4 year 0 1 1 2019 2019 2019 2019
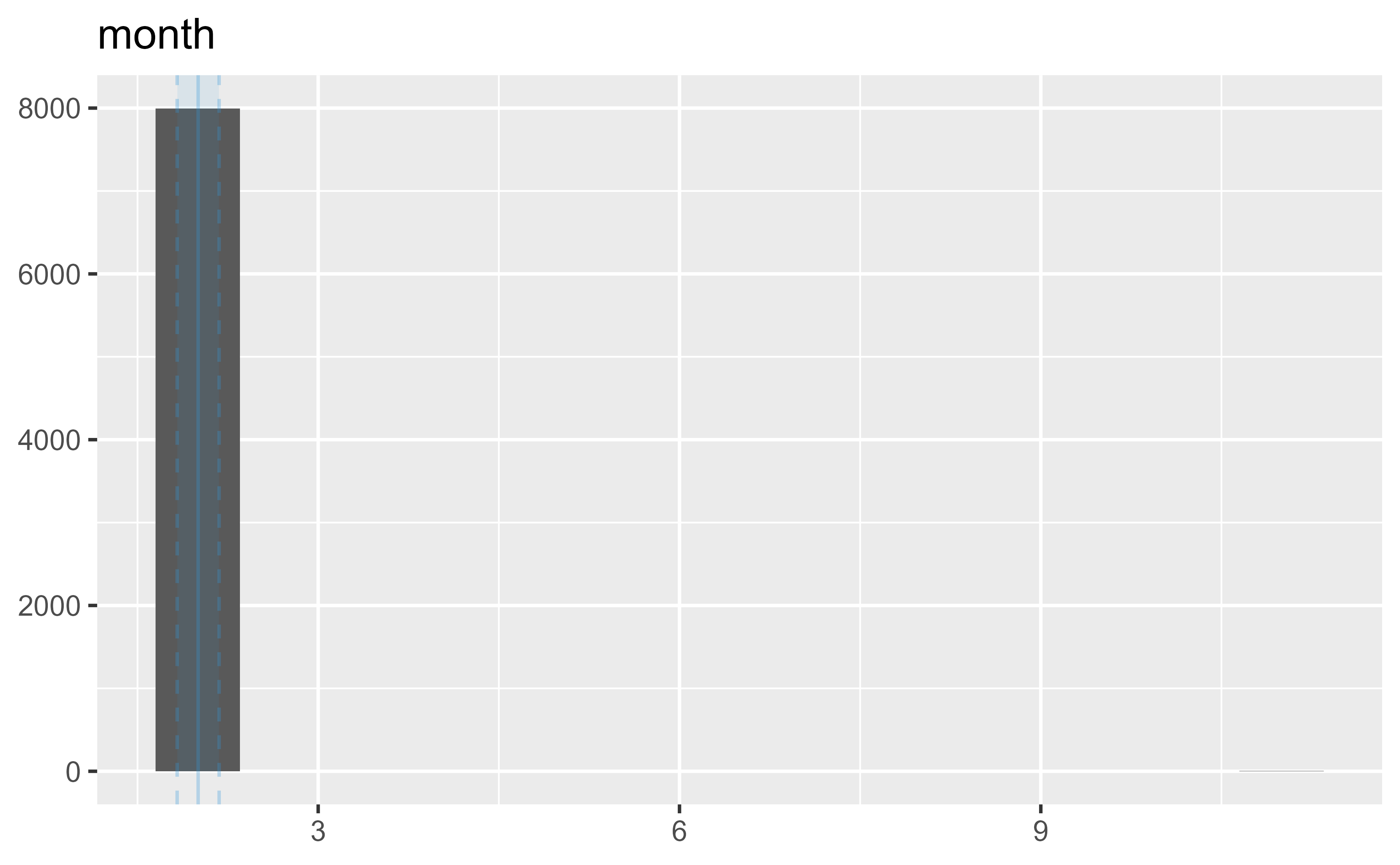
5 month 0 1 2 2 2 2 2
6 amp_wt_avg 0 1 1235 0.75 0.0019 0.049 0.12
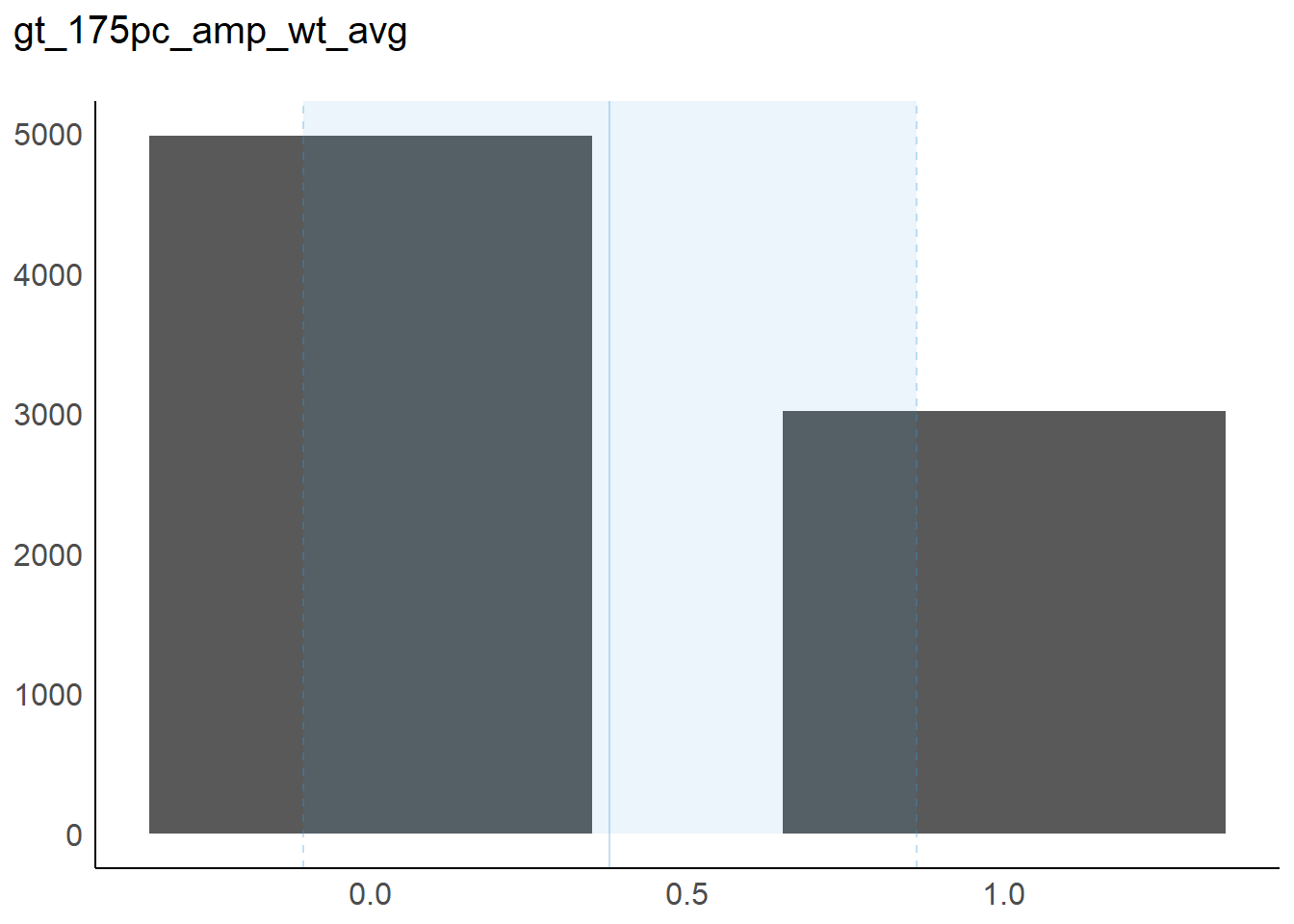
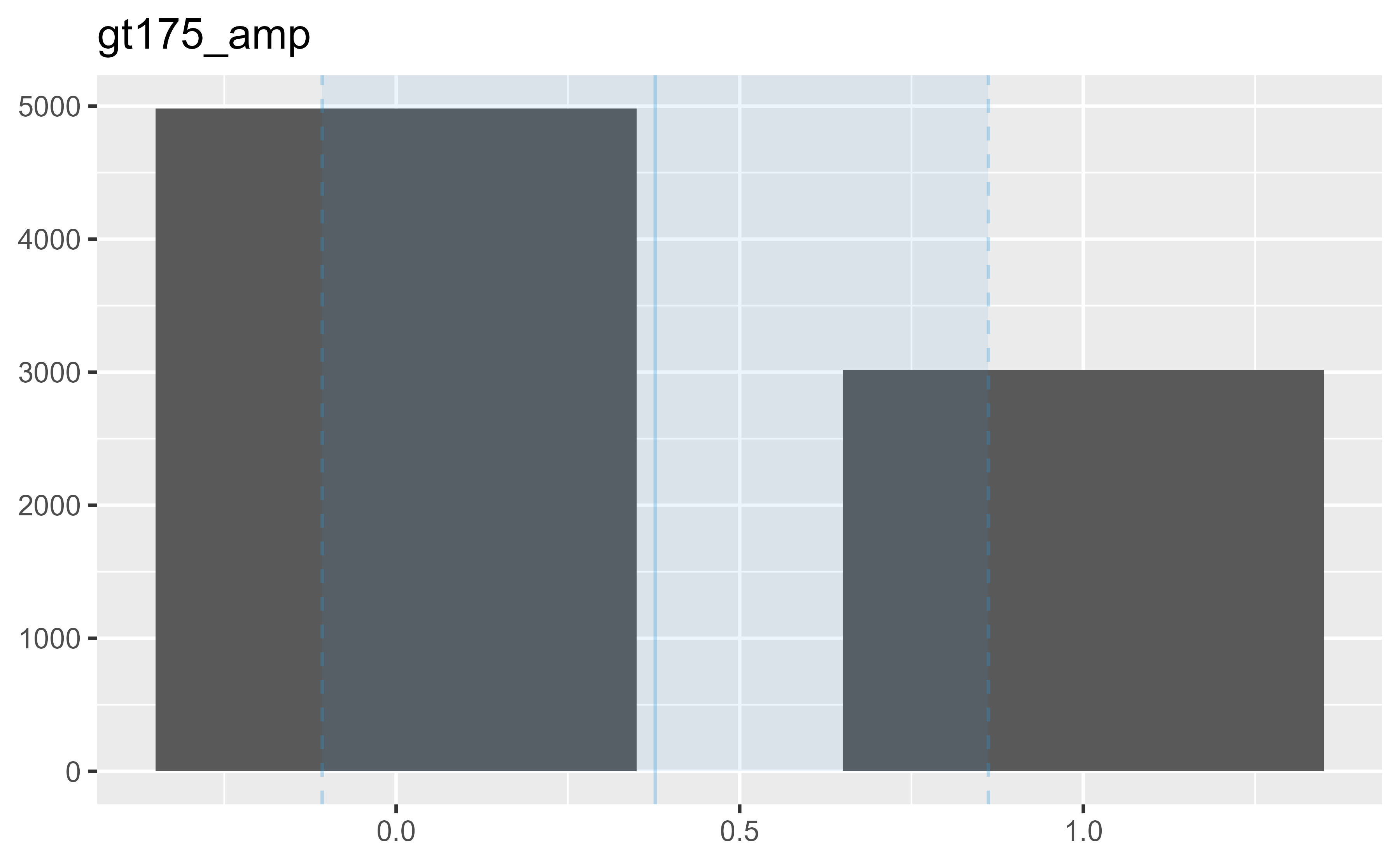
7 gt175_amp 0 1 2 0.38 0 0 0
p75 p100 iqr sd hist
1 0.84 127.3 0.74 5.56 ▇▁▁▁▁
2 100 28000 70 557.17 ▇▁▁▁▁
3 1 1 0 0.094 ▁▁▁▁▇
4 2019 2019 0 0 ▁▁▇▁▁
5 2 11 0 0.17 ▇▁▁▁▁
6 0.42 72.7 0.37 3.08 ▇▁▁▁▁
7 1 1 1 0.48 ▇▁▁▁▅
----- Categorical -----
col n_missng p_complt n_unique n_levels min
1 product_group 0 1 1237 NA 10036
2 ingredient 0 1 466 NA ABACAVIR SULFATE
3 strength 0 1 389 NA .025MG/24H
4 dosage 0 1 66 NA ADH. PATCH
5 route 0 1 14 14 DENTAL
6 mdr_unit_type 0 1 6 6 CAP
7 ndc 0 1 8000 NA 00002-3231-30
max
1 992
2 ZONISAMIDE
3 90MG
4 VIAL
5 VAGINAL
6 TDP
7 99207-0010-10
Distribution
Variable | Mean | SD | IQR | Range | Skewness
-----------------------------------------------------------------------
aca_ful | 1.43 | 5.56 | 0.74 | [3.28e-03, 127.26] | 13.10
package_size | 227.45 | 557.17 | 70.00 | [1.00, 28000.00] | 21.21
arated | 0.99 | 0.09 | 0.00 | [0.00, 1.00] | -10.48
year | 2019.00 | 0.00 | 0.00 | [2019.00, 2019.00] |
month | 2.00 | 0.17 | 0.00 | [2.00, 11.00] | 51.62
amp_wt_avg | 0.75 | 3.08 | 0.37 | [1.87e-03, 72.72] | 13.91
gt175_amp | 0.38 | 0.48 | 1.00 | [0.00, 1.00] | 0.51
Variable | Kurtosis | n | n_Missing
------------------------------------------
aca_ful | 236.32 | 8000 | 0
package_size | 849.35 | 8000 | 0
arated | 107.75 | 8000 | 0
year | | 8000 | 0
month | 2663.33 | 8000 | 0
amp_wt_avg | 264.56 | 8000 | 0
gt175_amp | -1.74 | 8000 | 0